News & Notice
NEOSSANCE SQUALANE USP(미국약전) 등재
Amyris Aprinnova Joint Venture Launches Pharmaceutical Grade "Neossance Squalane" USP
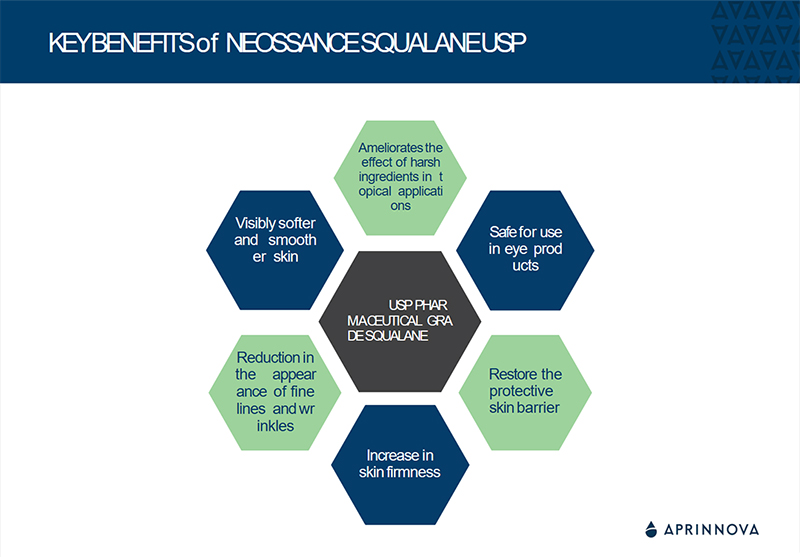
Squalane Monograph USP-NF36 REVISION AT A GLANCE
New definition has been added to Squalane Monograph USP41-NF36 by USP
- ‘’Squalane derived from β-farnesene, a fermentation product of plant sugars’’
Publication of new monograph
- The USP incorporation will be published on August 1, 2018 in the First Supplement to USP41-NF36.
Marketing
- Neossance Squalane USP can be commercially represented as USP Grade commencing February 1, 2018 under Early Adoption as authorized by Standard USP36
– General Notices and Requirements, subsection 3.10. Applicability of Standards.
What about EU?
- A USP ingredient can be used in an EU market OTC medicinal product. The new composition need to obtain the pre-market approval required by the Code on medicinal products. The pre-market approval must be confirmed or obtained by the responsible for marketing such product, who must be established in the EU.
New definition has been added to Squalane Monograph USP41-NF36 by USP
- ‘’Squalane derived from β-farnesene, a fermentation product of plant sugars’’
Publication of new monograph
- The USP incorporation will be published on August 1, 2018 in the First Supplement to USP41-NF36.
Marketing
- Neossance Squalane USP can be commercially represented as USP Grade commencing February 1, 2018 under Early Adoption as authorized by Standard USP36
– General Notices and Requirements, subsection 3.10. Applicability of Standards.
What about EU?
- A USP ingredient can be used in an EU market OTC medicinal product. The new composition need to obtain the pre-market approval required by the Code on medicinal products. The pre-market approval must be confirmed or obtained by the responsible for marketing such product, who must be established in the EU.